Description
Product details
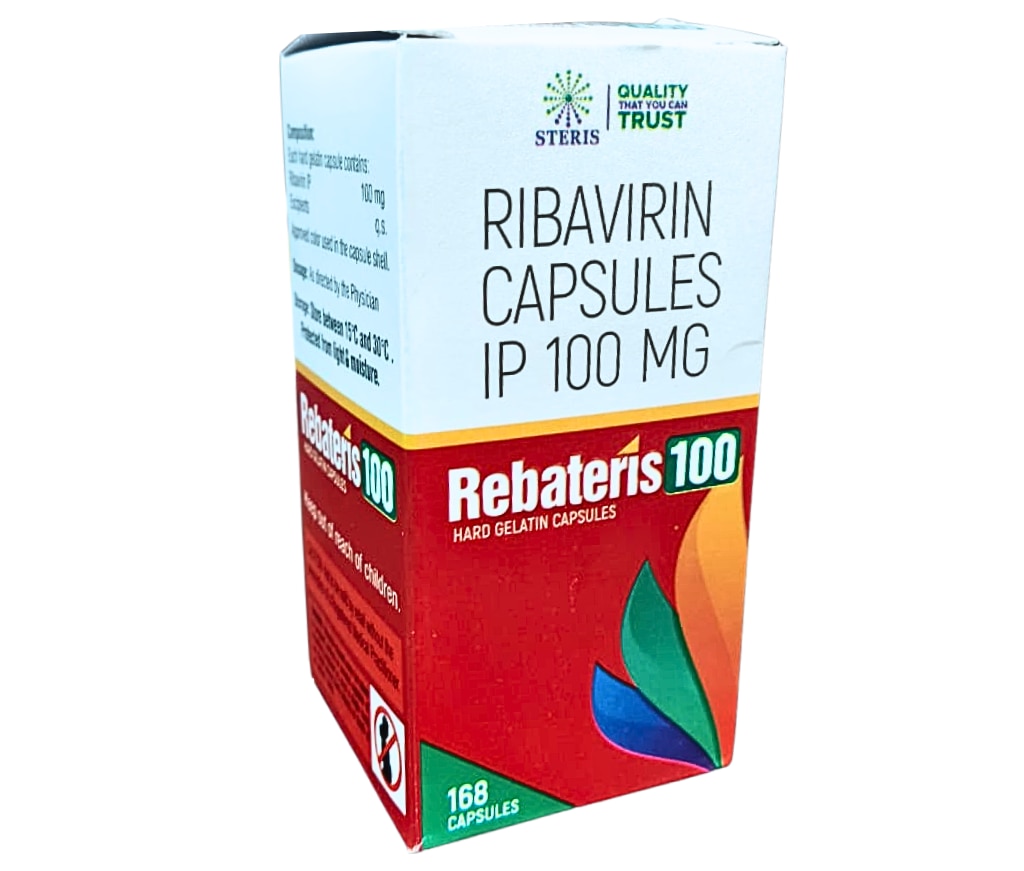
REBATERIS 100 contains Ribavirin IP 100mg in a hard gelatin capsule filled with white to off-white powder for optimal stability and bioavailability. Each pack typically includes 30 capsules, designed for oral administration with food to enhance absorption and reduce gastrointestinal upset. Ribavirin, a synthetic guanosine nucleoside analog, interferes with viral RNA polymerase and mRNA capping, halting hepatitis C virus (HCV) replication at multiple stages. This broad-spectrum antiviral also shows activity against respiratory syncytial virus (RSV) in select pediatric cases, though its primary indication remains HCV genotype management. The 100mg strength allows for weight-based dosing adjustments, making REBATERIS 100 suitable for adults and children over 3 years under specialist supervision. Unlike higher 200mg capsules, it supports finer dose reductions during therapy to manage side effects, ensuring better tolerability. Stored at room temperature away from moisture, it maintains potency for extended shelf life, aligning with pharmacopeial standards for purity and dissolution. Key Uses REBATERIS 100 treats chronic hepatitis C virus infection in combination therapies, particularly for patients ineligible for all-oral direct-acting antivirals. It boosts sustained virologic response rates when paired with pegylated interferon alfa, addressing genotypes 1 through 6 based on regional guidelines. In severe RSV lower respiratory tract infections, off-label use occurs in hospitalized infants, where aerosolized forms predominate, but oral REBATERIS 100 contributes to interferon combos for immunocompromised children. It curbs viral load, prevents liver fibrosis progression, and reduces cirrhosis risk, making it essential for long-term liver preservation. Additionally, emerging evidence supports its adjunct role in certain hemorrhagic fevers like Lassa virus, though availability limits widespread application. Always initiated by hepatologists, treatment durations span 24-48 weeks depending on genotype, viral load, and response milestones. Major Benefits REBATERIS 100 enhances viral clearance, achieving up to 50-80% sustained response rates in interferon combinations, significantly lowering hepatocellular carcinoma risk over five years. Patients experience normalized liver enzymes and fibrosis regression on biopsy follow-up. Its oral capsule form simplifies adherence compared to injectables, with twice-daily dosing fitting daily routines. The 100mg potency permits precise titration—e.g., 800-1400mg daily divided—for personalized therapy, minimizing excess exposure in lighter patients. Long-term, it preserves liver function, averting transplants and improving quality of life metrics like fatigue reduction and work productivity. Cost-effective for resource-limited settings, REBATERIS 100 bridges gaps until pan-genotypic regimens become accessible. Benefit Mechanism Clinical Impact Viral Suppression RNA polymerase inhibition >50% SVR in combos Liver Protection Fibrosis halt Reduced cirrhosis by 30-40% Flexible Dosing 100mg increments Better tolerability, adherence Broad Genotype Coverage Multi-genotype efficacy Versatile for diverse patients Potential Side Effects Common side effects include hemolytic anemia, peaking at weeks 4-8, necessitating hemoglobin monitoring and dose cuts to 600mg daily if levels drop below 10g/dL. Fatigue, headache, and insomnia affect over 40% of users, often resolving post-therapy. Gastrointestinal issues like nausea, diarrhea, and anorexia occur in 20-30%, mitigated by food intake. Dermatologic reactions—rash, pruritus—or flu-like symptoms from interferon pairing demand vigilance. Teratogenicity risks are high; dual contraception is mandatory for 6 months post-treatment due to sperm/ovum mutagenicity. Serious risks encompass pulmonary infiltrates, cardiac ischemia in vulnerable patients, and thyroid dysfunction. Avoid in hemoglobinopathies, severe renal impairment (CrCl <50mL/min), or pregnancy. Regular blood counts and ophthalmologic exams guide safe continuation. Dosage Guidelines Dosing is weight-based: adults <75kg receive mg/day (10 capsules: 5 AM, 5 PM); ≥75kg get 1200 mg/day (12 capsules: 6 AM, 6 PM), swallowed whole with meals. Pediatrics (≥3 years): 15mg/kg/day divided BID, rounded to the nearest 100mg via REBATERIS 100 capsules. Reduce by 200-400mg for anemia or neutropenia; discontinue if unresolved. Duration: 48 weeks for genotype 1, 24 weeks for others, with viral load checks at week 12. Swallow intact; no crushing. Conclusion REBATERIS 100 empowers hepatitis C management with proven antiviral potency and dosing precision from Steris Healthcare. It transforms prognosis for countless patients, but demands specialist oversight amid monitoring needs