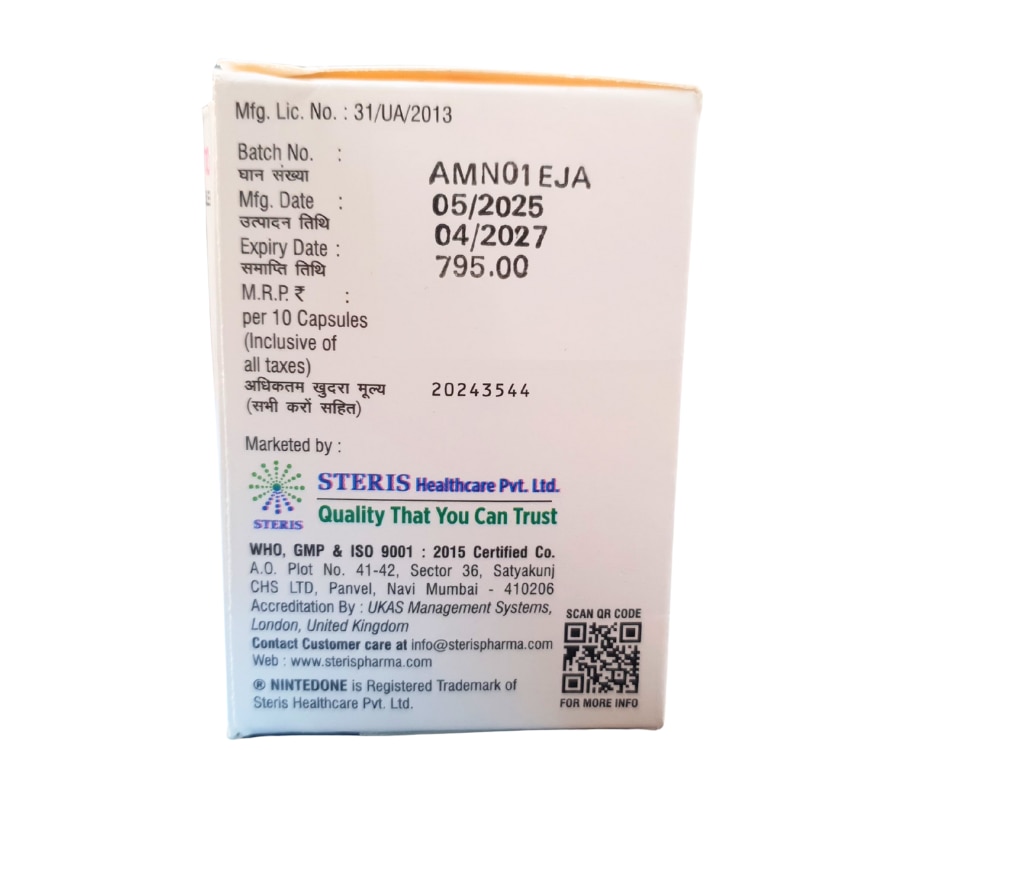
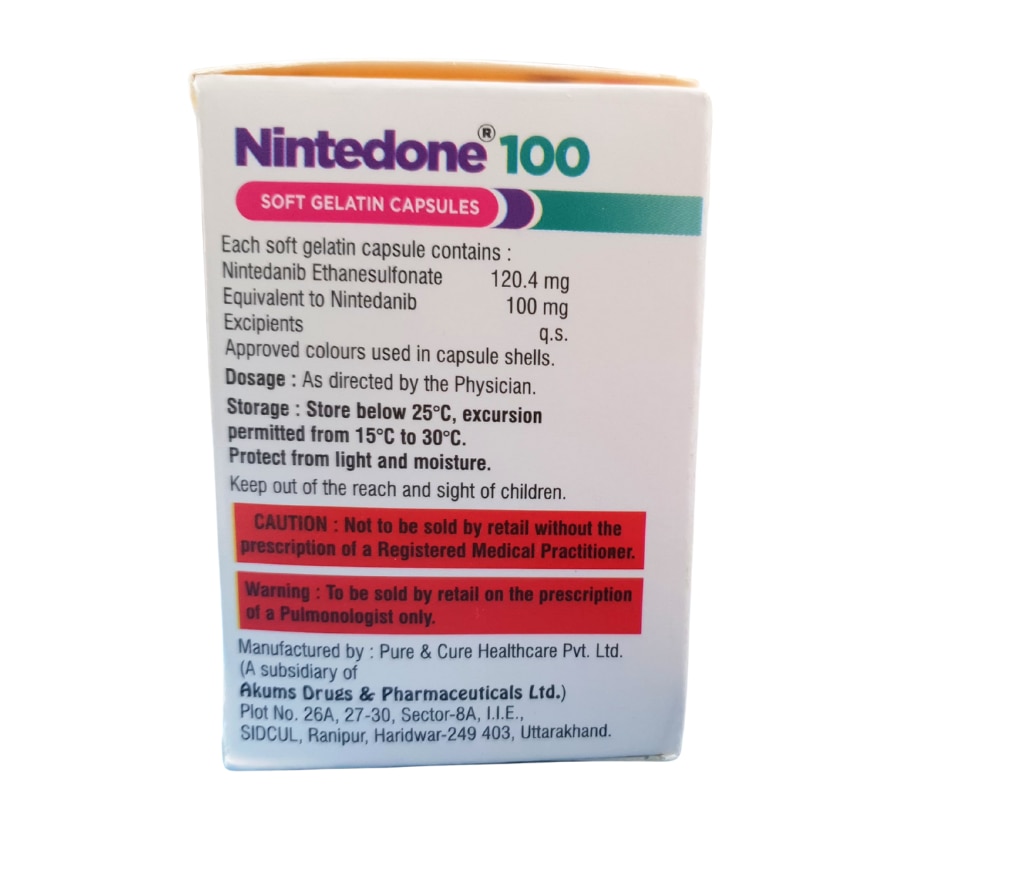
Description
Product details
NINTEDONE 100 Nintedanib 100 mg is a cutting-edge oral medication designed to combat progressive lung conditions and certain cancers, offering hope to patients facing debilitating fibrotic diseases. As a small-molecule tyrosine kinase inhibitor, it targets key pathways involved in abnormal cell growth and tissue scarring, making it a vital therapy for idiopathic pulmonary fibrosis (IPF), systemic sclerosis-associated interstitial lung disease (SSc-ILD), and progressive fibrosing interstitial lung diseases (PF-ILD). Manufactured to high standards, this formulation delivers precise dosing in a convenient capsule form, ensuring consistent absorption and efficacy for long-term management. Uses of Nintedanib 100 mg Nintedanib 100 mg stands out for its approved indications in slowing the decline of lung function in adults with IPF, a chronic and fatal lung disease characterized by progressive scarring of lung tissue. It is also indicated for SSc-ILD, where it helps manage lung fibrosis associated with systemic sclerosis, and PF-ILD, covering other chronic fibrosing lung conditions with a progressive phenotype. Beyond pulmonary fibrosis, Nintedanib has shown utility in oncology, particularly when combined with docetaxel for second-line treatment of advanced non-small cell lung cancer (NSCLC) of adenocarcinoma tumor histology, inhibiting tumor angiogenesis and growth. This medication addresses the underlying fibrotic processes by restricting fibroblast proliferation and migration, which are central to lung tissue damage. In clinical practice, physicians prescribe Nintedanib 100 mg for patients with confirmed diagnoses via high-resolution CT scans or lung biopsies, often after other treatments fail. Its versatility extends to off-label considerations in other cancers like pancreatic or colorectal, though primary use remains in respiratory fibrosis and lung cancer, providing a targeted approach where traditional therapies fall short. Key Benefits of Nintedanib 100 mg One of the primary benefits of Nintedanib 100 mg is its proven ability to reduce the rate of forced vital capacity (FVC) decline, a key measure of lung function, by up to 50% in IPF patients compared to placebo in landmark trials. This translates to slower disease progression, fewer acute exacerbations, and improved quality of life, allowing patients to maintain daily activities longer. The oral administration enhances patient compliance, avoiding the need for infusions or hospital visits. Slows Fibrosis Progression: By inhibiting multiple tyrosine kinases, it curbs excessive scar tissue formation in the lungs, preserving respiratory capacity over time. Anti-Angiogenic Effects: In cancer settings, it blocks vascular endothelial growth factor receptors (VEGFR), reducing tumor blood supply and metastasis risk. Broad-Spectrum Kinase Inhibition: Targets fibroblast growth factor receptors (FGFR), platelet-derived growth factor receptors (PDGFR), and others, addressing multifaceted disease pathways. Manageable Long-Term Use: Clinical data supports sustained benefits with dose adjustments, minimizing hospitalizations for respiratory failure. Improved Survival Metrics: In combination therapies for NSCLC, it extends progression-free survival, offering valuable time for patients. These advantages make Nintedanib 100 mg a cornerstone in modern pulmonology and oncology, backed by phase III trials demonstrating statistically significant outcomes in diverse patient populations. Side Effects of Nintedanib 100 mg While effective, Nintedanib 100 mg can cause gastrointestinal side effects as the most common issue, affecting over 60% of users primarily due to its impact on kinase signaling in the gut. Diarrhea often starts within the first two weeks and is typically manageable with dose reduction or antidiarrheal agents like loperamide. Other frequent effects include nausea, vomiting, and decreased appetite, which may lead to weight loss if not addressed. More serious side effects warrant close monitoring: Liver Enzyme Elevations: Hepatotoxicity occurs in about 10-15% of patients, necessitating monthly liver function tests, especially in those with pre-existing liver conditions. Cardiovascular Risks: Potential for arterial thromboembolism or hypertension; baseline cardiac evaluation is recommended. Bleeding Events: Due to VEGF inhibition, minor bleeding like epistaxis is common, but gastrointestinal hemorrhage requires immediate attention. Rare but Severe: Interstitial lung disease progression (paradoxical worsening), perforations, or severe infections in immunocompromised patients. Patients should report persistent diarrhea, abdominal pain, jaundice, or unusual fatigue promptly. Contraindications include pregnancy (category D), active bleeding, or hypersensitivity, with strong recommendations against use in moderate to severe hepatic impairment. Dosage and Administration Insights Typically initiated at 150 mg twice daily for IPF and ILD, Nintedanib 100 mg allows for flexible dosing—starting lower at 100 mg BID if tolerated poorly, with food to reduce GI upset. For NSCLC, it's 200 mg BID with docetaxel cycles. Regular monitoring every 2-3 months adjusts for tolerance, emphasizing adherence to slow disease modification. Conclusion Nintedanib 100 mg represents a breakthrough in managing progressive fibrotic lung diseases and select cancers, delivering targeted inhibition of fibrotic and angiogenic pathways for tangible clinical benefits. Its ability to slow FVC decline, enhance patient quality of life, and integrate into combination regimens underscores its value, despite manageable side effects like diarrhea that respond well to supportive care. For those battling IPF, SSc-ILD, or NSCLC, Nintedanib 100 mg offers a proactive defense against relentless progression, empowering longer, more active lives under specialist guidance.