Description
Product details
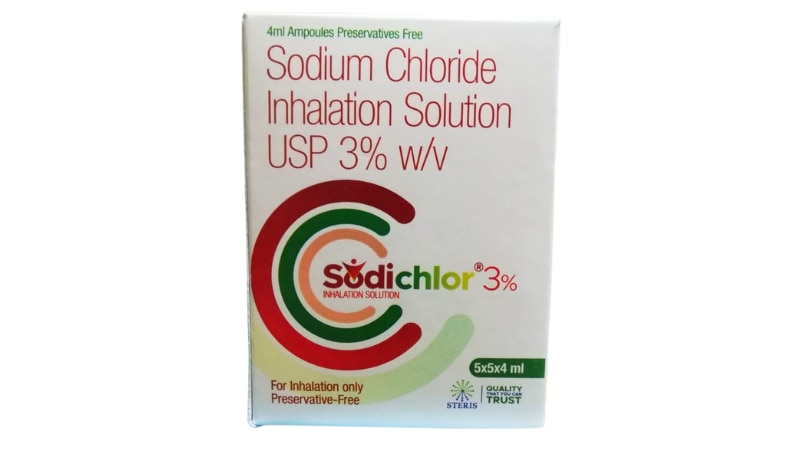
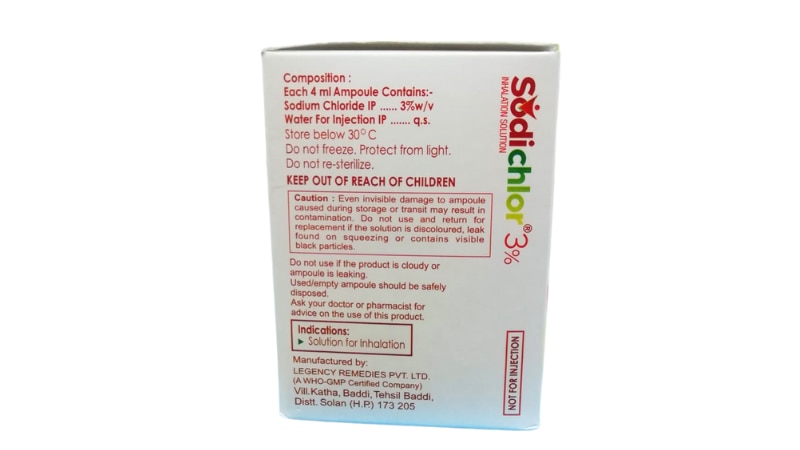
SODICHLOR 3% is a sterile, preservative-free hypertonic saline inhalation solution specifically formulated to help manage respiratory conditions characterized by thick, tenacious mucus secretions. This pharmaceutical-grade formulation contains 3% sodium chloride in purified water, offering a concentration three times higher than normal saline, designed to work through osmotic principles to improve airway clearance and respiratory function. As a trusted therapeutic agent in respiratory care, SODICHLOR 3% represents a non-antibiotic approach to managing chronic respiratory conditions, making it an invaluable tool for patients suffering from cystic fibrosis, chronic obstructive pulmonary disease (COPD), bronchiectasis, and other conditions where mucus clearance is compromised. The solution is administered via nebulization, allowing the medication to reach deep into the bronchial passages where it can exert its therapeutic effects most effectively. How SODICHLOR 3% Works The mechanism of action behind SODICHLOR 3% is elegantly simple yet highly effective. When nebulized and inhaled, the hypertonic saline solution creates an osmotic gradient in the airways. This means that the higher salt concentration in the solution draws water from the surrounding tissues into the airway surface liquid layer. This influx of water hydrates the mucus, making it less viscous and sticky, which significantly improves the ability to cough up and clear secretions from the lungs. Additionally, SODICHLOR 3% helps restore the normal salt and water balance on airway surfaces, which is particularly important in conditions like cystic fibrosis where this balance is naturally disrupted. The solution also stimulates ciliary beat frequency, enhancing the natural cleaning mechanism of the respiratory tract. By improving mucociliary clearance, SODICHLOR 3% helps reduce the bacterial burden in the airways, potentially decreasing the frequency of respiratory infections and exacerbations. Primary Uses and Applications SODICHLOR 3% is prescribed for various respiratory conditions where enhanced mucus clearance is clinically beneficial: Cystic Fibrosis Management: Perhaps the most well-established use, SODICHLOR 3% has become a cornerstone therapy for patients with cystic fibrosis. Regular use helps thin the abnormally thick mucus characteristic of this genetic condition, improving lung function and quality of life while reducing the risk of pulmonary exacerbations. Chronic Obstructive Pulmonary Disease: For COPD patients who struggle with excessive mucus production and poor clearance, SODICHLOR 3% provides relief by facilitating easier expectoration and improving breathing comfort. Bronchiectasis: This condition, characterized by permanently damaged and widened airways that trap mucus, benefits significantly from hypertonic saline therapy. SODICHLOR 3% helps clear the pooled secretions that contribute to recurrent infections. Post-Operative Respiratory Care: Following certain surgical procedures, particularly thoracic surgeries, SODICHLOR 3% may be used to prevent mucus plugging and atelectasis by maintaining adequate airway hydration. Sputum Induction: In diagnostic settings, SODICHLOR 3% can be used to induce sputum production for laboratory testing when patients cannot spontaneously produce adequate samples. Key Benefits of SODICHLOR 3% The therapeutic advantages of SODICHLOR 3% extend across multiple dimensions of respiratory health: Enhanced Mucus Clearance: The primary benefit is the dramatic improvement in the ability to clear thick, sticky mucus from the airways. Patients often report easier breathing and more productive coughing within minutes of treatment. Improved Lung Function: Clinical studies have demonstrated that regular use of hypertonic saline solutions like SODICHLOR 3% can lead to measurable improvements in pulmonary function tests, including forced expiratory volume (FEV1) and forced vital capacity (FVC). Reduced Infection Frequency: By promoting better mucus clearance and reducing bacterial colonization in the airways, SODICHLOR 3% may help decrease the frequency of respiratory infections and disease exacerbations, leading to fewer hospitalizations. Non-Antibiotic Therapy: In an era of increasing antibiotic resistance, SODICHLOR 3% offers a mechanical rather than chemical approach to managing respiratory symptoms, making it a valuable addition to any treatment regimen without contributing to resistance patterns. Cost-Effective Treatment: Compared to many specialized respiratory medications, SODICHLOR 3% represents a relatively affordable therapeutic option that can be used long-term without prohibitive expense. Quality of Life Enhancement: Patients using SODICHLOR 3% regularly often report significant improvements in their daily activities, exercise tolerance, and overall sense of well-being due to easier breathing and reduced respiratory distress. Compatibility with Other Treatments: SODICHLOR 3% can be safely used alongside other respiratory medications, including bronchodilators, corticosteroids, and antibiotics, as part of a comprehensive treatment plan. Potential Side Effects and Considerations While SODICHLOR 3% is generally well-tolerated, patients should be aware of potential side effects: Common Side Effects: The most frequently reported adverse effects include throat irritation, coughing (especially initially), unpleasant taste, and mild wheezing or chest tightness. These effects are typically transient and often diminish with continued use as the body adapts to the therapy. Bronchospasm: Some patients, particularly those with hyperreactive airways or asthma, may experience bronchospasm (sudden narrowing of the airways) during or after treatment. This is why healthcare providers often recommend using a bronchodilator before SODICHLOR 3% administration. Temporary Oxygen Desaturation: In some cases, especially in patients with severe lung disease, temporary decreases in blood oxygen levels may occur immediately after treatment, though levels typically return to baseline within 30 minutes. Nasal Congestion and Rhinorrhea: Some patients may experience nasal stuffiness or runny nose following inhalation therapy. Less Common Effects: Headache, nausea, dizziness, or voice changes may occasionally occur but are generally mild and self-limiting. Patients with certain conditions such as uncontrolled hypertension, severe electrolyte imbalances, or recent hemoptysis should use SODICHLOR 3% only under close medical supervision. As with any medical treatment, it's essential to discuss your complete medical history with your healthcare provider before beginning therapy. Conclusion SODICHLOR 3% represents a scientifically sound, clinically proven approach to managing respiratory conditions complicated by thick mucus secretions. Its mechanism of action—leveraging osmotic principles to hydrate and mobilize airway secretions—addresses a fundamental problem faced by millions of patients with chronic respiratory diseases. The substantial body of evidence supporting its efficacy, combined with its favorable safety profile and cost-effectiveness, makes SODICHLOR 3% an indispensable component of modern respiratory care.