Description
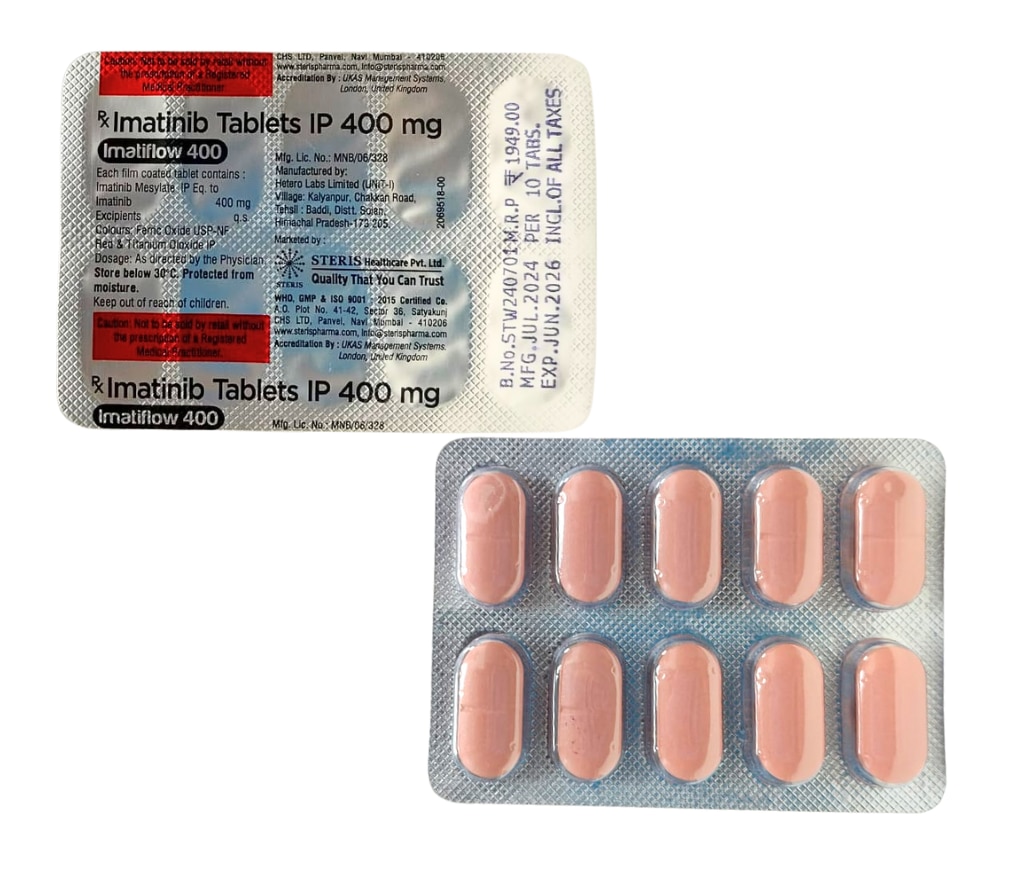
Product details
IMATIFLOW 400, containing Imatinib IP 400mg, represents a breakthrough in targeted cancer therapy, offering patients a precise weapon against specific malignancies. This oral medication belongs to the class of tyrosine kinase inhibitors, revolutionizing treatment for conditions once deemed challenging to manage. By blocking abnormal proteins that fuel cancer cell growth, IMATIFLOW 400 helps restore normal cellular function and improves quality of life for many users. Product Overview IMATIFLOW 400 is a branded formulation of Imatinib mesylate 400mg, produced to high pharmaceutical standards for consistent efficacy and bioavailability. Each tablet delivers the active ingredient in a film-coated form, designed for once-daily administration with food to enhance absorption and minimize gastrointestinal upset. Developed for adult patients, it targets cancers driven by dysregulated tyrosine kinases like BCR-ABL, c-KIT, and PDGFR, making it a cornerstone in modern oncology protocols. The medication's mechanism hinges on competitive inhibition at the ATP-binding site of these kinases, halting downstream signaling pathways that promote uncontrolled proliferation, survival, and metastasis of malignant cells. Unlike traditional chemotherapy, which broadly attacks dividing cells, IMATIFLOW 400 spares most healthy tissues, leading to a more favorable safety profile. Clinical data from pivotal trials underscore its role as first-line therapy, with high response rates observed within months of initiation. Primary Uses IMATIFLOW 400 excels in treating chronic myeloid leukemia (CML), particularly in the chronic phase where it induces deep molecular responses in over 80% of patients. It suppresses the Philadelphia chromosome-positive cells hallmark of CML by inhibiting the BCR-ABL fusion protein, dramatically extending progression-free survival. For newly diagnosed patients, standard dosing starts at 400mg daily, often achieving complete cytogenetic remission. In gastrointestinal stromal tumors (GIST), IMATIFLOW 400 targets KIT or PDGFRA mutations, shrinking unresectable tumors and delaying progression in advanced cases. The recommended dose remains 400mg once daily, with potential escalation to 600-800mg for resistant strains, as per oncology guidelines. It also finds application in dermatofibrosarcoma protuberans (DFSP), aggressive systemic mastocytosis, and certain myelodysplastic syndromes, broadening its utility in precision medicine. Healthcare providers monitor response via PCR for BCR-ABL transcripts in CML or imaging for GIST, adjusting therapy based on milestones like major molecular response. Patients with Ph+ acute lymphoblastic leukemia (ALL) may receive it adjunctively, enhancing outcomes when combined with chemotherapy. Overall, IMATIFLOW 400 transforms these diseases from fatal to chronically manageable. Key Benefits One standout benefit of IMATIFLOW 400 is its oral convenience, eliminating the need for frequent hospital visits associated with infusions. Response rates exceed 90% in early CML, with many patients achieving treatment-free remission after sustained deep responses, a paradigm shift from interferon-era therapies. Tumor control in GIST prevents life-threatening complications like bowel obstruction, improving survival from months to years. Its specificity reduces severe toxicities, allowing patients to maintain daily activities, work, and social engagements with minimal disruption. Long-term studies report 10-year survival rates above 80% in CML, underscoring durable efficacy. Additional advantages include cost-effectiveness over biologics and compatibility with supportive care like hydroxyurea for cytoreduction. Fluid retention, while common, responds well to diuretics, preserving cardiac function. For Indian patients, accessible pricing via brands like IMATIFLOW 400 supports equitable care in resource-limited settings. Potential Side Effects While generally well-tolerated, IMATIFLOW 400 can cause edema (swelling in legs or face) in up to 70% of users, managed by dose reduction or supportive measures. Gastrointestinal issues like nausea, vomiting, and diarrhea affect 40-50%, often resolving with antiemetics and dietary adjustments. Musculoskeletal pain, cramps, and fatigue occur frequently, alongside rash and abdominal discomfort. Hematologic effects include neutropenia or thrombocytopenia, necessitating regular blood counts. Rare but serious risks involve liver enzyme elevation, heart failure, or hemorrhage; prompt reporting of symptoms like shortness of breath or yellowing skin is crucial. Avoid grapefruit juice, as it elevates drug levels, and inform doctors of concurrent medications like CYP3A4 inhibitors. Pregnancy category D status advises contraception, with monitoring for growth retardation in exposed fetuses. Dizziness may impair driving, so caution is essential during initial weeks. Dosage and Administration Guidelines Swallow IMATIFLOW 400 whole with a meal and full glass of water to optimize pharmacokinetics and reduce esophageal irritation. The standard adult dose for CML chronic phase is 400mg daily; for accelerated phase or blast crisis, 600mg. GIST dosing mirrors 400mg, titrated based on tolerance and response. Renal or hepatic impairment requires dose adjustments—reduce to 300-400mg in moderate cases. Do not crush or chew tablets. Missed doses should be taken promptly unless near the next, avoiding doubles. Long-term use demands quarterly monitoring of CBC, liver function, and ECG. Precautions and Interactions Patients with a cardiac history need baseline echocardiograms, as QT prolongation or effusion risks exist. Drug interactions abound: St. John's wort induces metabolism, reducing efficacy; ketoconazole boosts levels. Vaccines, especially live ones, are contraindicated during therapy. Regular dermatologic checks mitigate skin cancer risk from prolonged use. Hydration combats fluid retention, and low-salt diets aid management. Steris Healthcare emphasizes patient education via counseling on adherence for optimal outcomes. Conclusion IMATIFLOW 400 (Imatinib IP 400mg) stands as a testament to targeted therapy's power, offering robust control over CML, GIST, and related cancers with a balance of efficacy and manageability. Its benefits in prolonging life and enhancing daily functioning far outweigh transient side effects for most when monitored diligently. Consult oncologists for personalized plans, ensuring this innovative treatment maximizes hope and health in the fight against cancer.